Driving Innovation, Transforming Lives
At Caring Cross, our commitment to research and development (R&D) is the cornerstone of our mission to improve health equity. We relentlessly focus on driving innovation to push the boundaries of the application of medical science to human health.
Our team is comprised of veterans in the gene therapy field, with senior members each having decades of experience.
We focus our efforts on the following research and development activities:
Molecular research innovation
Molecular vectors are used to deliver therapeutic genes into cells. We are focusing our efforts on Lentiviral (LV) and Adeno Associated viral (AAV) vectors. These vectors have been shown to be safe and efficacious, with several LV and AAV-based products approved by the FDA.
Some of our R&D activities focus upon improving vector design, leading to higher titer vectors with optimized therapeutic gene expression and function.
We also focus upon optimizing therapeutic gene design and expression. One such class of therapeutic gene is the chimeric antigen receptor (CAR) that is transferred to T cells to kill target cells, for example, cancer cells. One area of research is developing highly specific binding domains where the CAR binds to target proteins on the surface of cancer cells. We have developed a human binding domain library that integrates a barcoding system and artificial intelligence/machine learning algorithms to optimize the binding characteristics of CARs to target cells. This leads to significant improvements in CAR function. In addition, we optimize overall CAR function by combining binding domains with appropriate co-stimulation domains and other elements to improve CAR safety, efficacy and T cell persistence.
Therapeutic genes other than CARs are also optimized for expression and function using our algorithms and extensive experience.
We leverage our knowledge and actively partner with companies to assist them with their CAR R&D activities. If you are interested in learning how these capabilities can help your company, please contact us at the link below.
Cell manufacturing innovation
Advanced Medicines like cellular gene therapies are transforming the lives of patients that receive them. The great majority of the products that have been approved are autologous, meaning that they are derived from patient’s cells that are removed from the patient, reprogrammed with a therapeutic gene via a vector, and then reintroduced back to the patient.
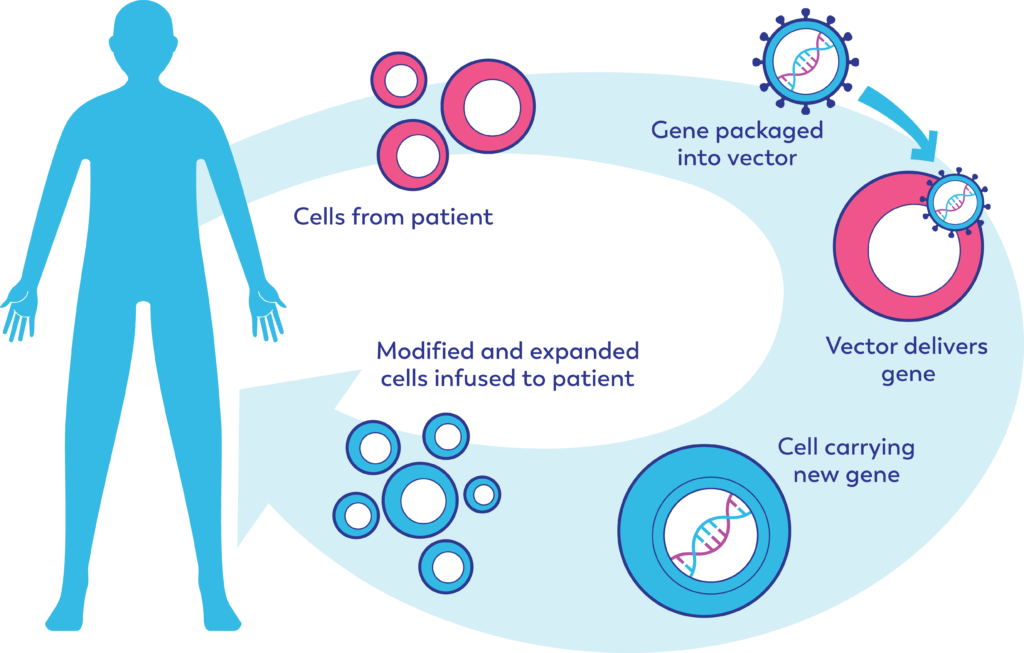
Autologous reprogrammed CAR-T cells and stem cells are derived from the patient’s own cells
Some of our R&D activities focus upon improving this autologous cell manufacturing process. One such advance is the development of a T-pure™ reagent that significantly simplifies and reduces the cost of the CAR-T cell manufacturing process. By simplifying the process, we increase its robustness and decrease the cost by avoiding failures that would otherwise occur with a more complex process.
One advantage of our process is that the starting material from the patient can be whole blood or apheresis blood product. Apheresis is expensive and having the option to generate CAR-T cells from blood significantly reduces the overall cost. This is important for patients in low- and middle-income countries where access to centers that perform apheresis is limited.
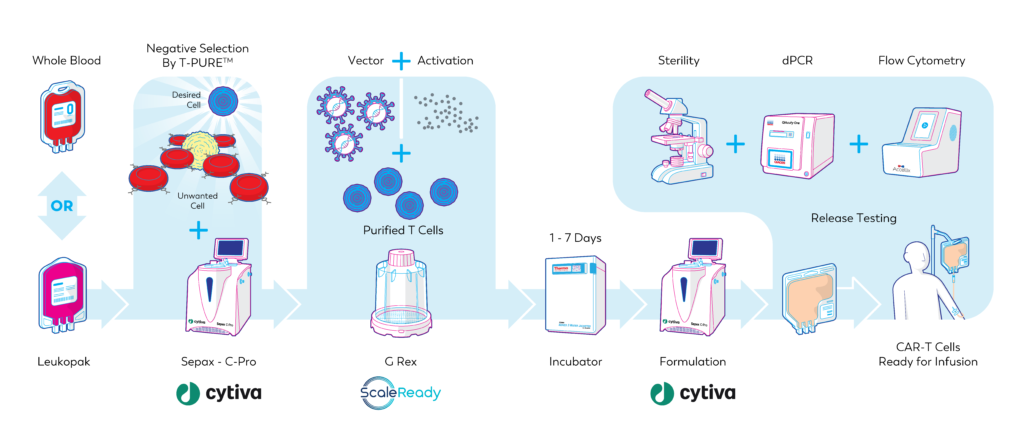
Our simplified and affordable manufacturing process for the production of CAR-T cells
Vector manufacturing innovation
Vectors are key critical materials that are used to reprogram cells with therapeutic genes. They are the single greatest material cost when producing cellular gene therapies. Improving vector manufacturing processes improves their quality and lowers their cost.
Our team has developed novel and improved LV and AAV manufacturing processes that show high titer and functionality.
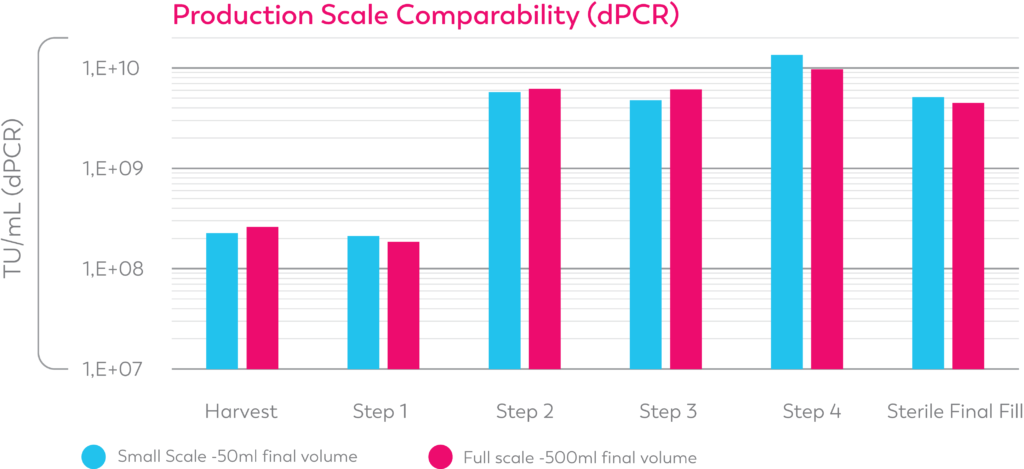
Our Lentiviral vector manufacturing process results in high titer LV product after sterile filtration.
Lentiviral vectors made using this process is now available to investigators in the gene therapy community though Vector BioMed, a CDMO which was spun-out from Caring Cross. The cost of GMP vectors produced by Vector BioMed are about half the price of competing CDMOs. For more information on Vector BioMed can create and manufacture GMP vectors expressing your specific therapeutic gene of interest, please visit www.vectorbiomed.com.
Collaborate with us on innovating the future of medicine
At Caring Cross, we believe that every innovation has the potential to transform lives.
Join us in our mission to push the boundaries of medical science, drive innovation, and bring hope—and action—to patients around the world.
Together, we can make a meaningful difference in the fight against disease and pave the way for a healthier, brighter future.
Contact us to learn more at [email protected].

